
Pipeline
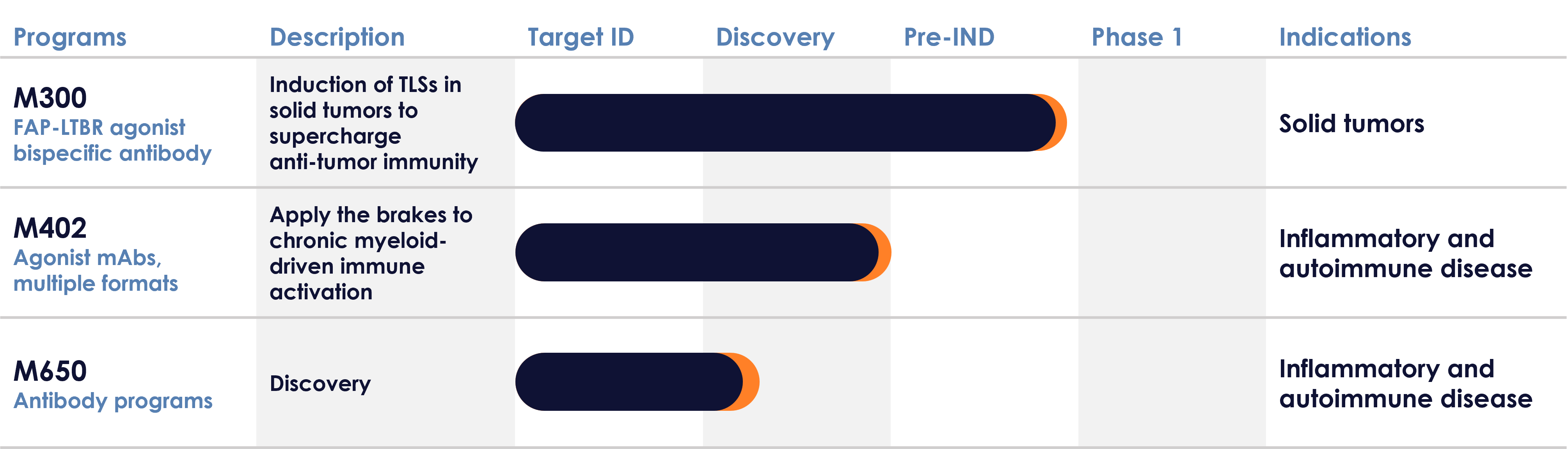
M300
Recently, the presence of Tertiary Lymphoid Structures (TLSs) in tumors has been shown to be strongly predictive of both significantly improved patient outcomes and better responses to therapy for cancer patients across multiple solid tumor types. These striking observations have been seen in several thousand cancer patients, indicating the remarkable therapeutic potential of inducing TLSs1,2,3,4. TLSs are aggregates of immune cells that form in tumor tissue as part of our bodies’ natural anti-cancer mechanisms, and drive powerful immune responses by recruiting, educating, and activating new anti-tumor T and B cells.
The M300 program is advancing a FAP-LTBR bispecific antibody, designed to conditionally agonize lymphotoxin beta receptor (LTBR) in the tumor, on co-engagement of fibroblast activation protein (FAP) expressed by cancer associated fibroblasts, to induce the formation of TLSs in solid tumors. Conditional LTBR agonism using a FAP-LTBR bispecific antibody promotes TLSs and drives robust immune cell recruitment and education, leading to potent anti-tumor responses in preclinical models [AACR 2024 Poster]. M300 has been developed to promote improved anti-tumor immune responses, enabling better outcomes for patients either alone or in combination with current treatments, and to provoke new anti-tumor responses in patients resistant to standard of care. An Investigational New Drug (IND) filing is anticipated in early 2026.
M402
M402 is a first-in-class stromal agonist antibody, designed to dampen down myeloid-driven biology in autoimmune and inflammatory disease. M402 targets an inhibitory receptor found on human immune cells, which acts as a brake on the immune system to regulate immune activity. Increasing the inhibitory function via receptor agonism in inflammatory disease patients, where immune cells are over-active, holds significant promise in the clinic. Our goal is to improve outcomes for inflammatory disease patients by dampening down the immune system’s overactive state5.
M402 is designed to inhibit specific immune cell populations, including myeloid cells, important drivers of auto-immune and inflammatory disease, often overlooked by the current standard of care. This program has the potential to benefit patients across inflammatory diseases including rheumatoid arthritis, lupus, Sjogrens syndrome, graft-versus-host disease and others.
References:
1) Fridman et al., Nature Reviews 2019
2) Vanhersecke et al., Nature Communications 2021
3) Cabrita et al., Nature 2020
4) Asrir et al., Nature 2022
5) Paluch et al., Frontiers in Immunology 2018